A radiocarbon determination is a measure of the amount of radiocarbon in the sample. When any organism is alive it continues to take up radiocarbon from the atmosphere, but once it has died the amount gradually declines because of radioactive decay.
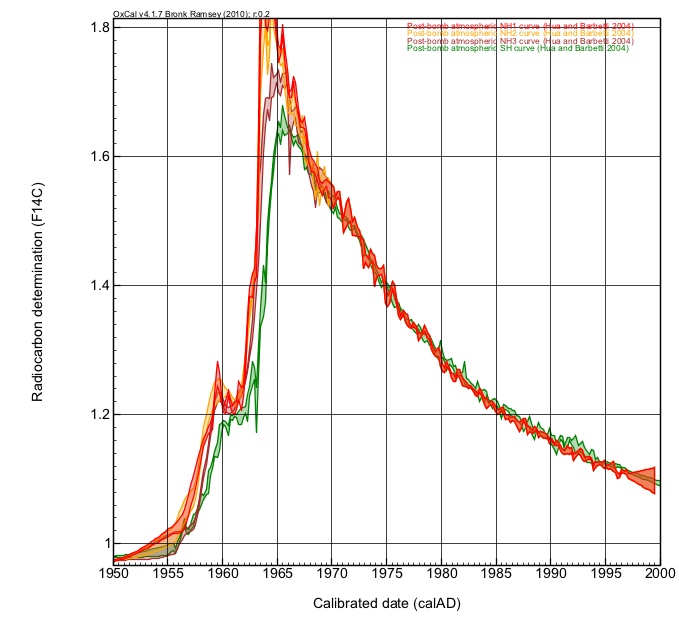
In the 1940s, the first atomic nuclear bombs were exploded. Radiocarbon (14C) is created artificially through this process. In the 1950s and 60s atmospheric testing saw large amounts of 'bomb' radiocarbon created, such that in the mid 1960s the radiocarbon in the atmosphere was double its natural amount. Since then, the level has declined as radiocarbon enters the biosphere. The illustration below shows atmospheric radiocarbon measurements, collected from three latitudinal stations in the northern hemisphere, and in the southern hemisphere, showing the precise concentration of atmospheric radiocarbon throughout the mid to late 20th century.

Calibration is performed by comparing the radiocarbon measurement (or measurements) made on your sample to these known age records of atmospheric radiocarbon. An example is given below.
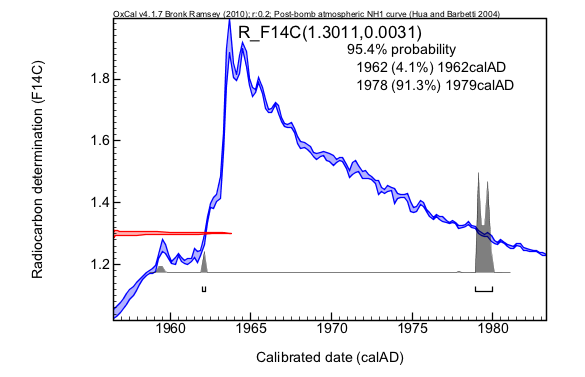
The main elements of this plot are:
The range of possible ages is also shown for other levels of confidence. We can be 68% sure that the sample dates to 1979. At 95% confidence we can be more than 90% confident that the sample dates between 1978-1979, but there is a small chance (4%) that the sample age dates from 1962.
See also Explanation of radiocarbon results.